Canadian researchers have developed an inhaled vaccine for COVID-19 that might not only protect people from this coronavirus, but also animal viruses that could one day infect humans.
The challenge: As viruses spread, they undergo minor genetic mutations, and when we discover a version of a virus with unique mutations, we call it a new strain. The coronavirus is no different — researchers discover new strains every week.
The problem is that the coronavirus’ spike protein is particularly susceptible to mutations. That’s the part of the virus targeted by the available vaccines, and the mutations are making the vaccines less effective.
In addition, antibody levels in the blood wane over time, reducing immunity to infection and requiring booster shots.
“The inhaled vaccine puts the immune system in our lungs on high alert for everything.”
Matt Miller
The inhaled vaccine: To overcome this problem, researchers at Canada’s McMaster University developed a vaccine that targets two parts of the virus that very rarely mutate, which are common to the whole family of coronaviruses, as well as the spike protein.
“By targeting a breadth of immune responses to different parts of the COVID virus, we expect to see broader protection,” researcher Fiona Smaill said in December 2021.
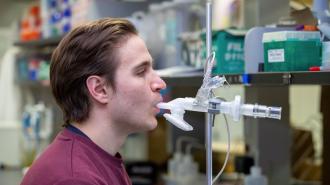
The vaccine is also designed to be inhaled, rather than injected. This creates a local immune response right in the airways where the virus first takes hold, called “mucosal immunity” — something vaccines injected into arm muscle don’t do very well.
“This inhaled version of the vaccine does something really special where it essentially puts the immune system in our lungs on high alert for everything,” researcher Matt Miller said. “It stimulates what’s called trained innate immunity, which is a way that our immune system can become more prepared to deal with any infection in the future.”
The trials: The McMaster researchers have now wrapped up a mouse study of their inhaled COVID-19 vaccine, and based on the newly published results, it works as hoped, triggering the production of antibodies and T cells, and stimulating trained innate immunity.
The researchers are now turning their attention to a phase 1 clinical trial, in which at least 30 people who’ve already received two doses of a COVID-19 vaccine by Pfizer or Moderna will get the inhaled vaccine as a booster.
The purpose of this trial will be to test the safety of the inhaled vaccine and see what sort of immune response it produces in participants’ lungs, airways, and blood. If the study is a success, larger human trials would be the next step on the path to approval.
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].