Novo Nordisk has published the results of its massive SELECT trial, which found that the weight-loss drug semaglutide (brand name Wegovy) reduced participants’ risk of a serious cardiovascular event — heart attack, stroke, and death from heart disease — by 20%.
Wegovy 101: In June 2021, the FDA approved Wegovy as a weight-loss treatment for people who have obesity or are overweight with another related condition, such as high cholesterol or high blood pressure.
Given all the health issues associated with carrying excess weight, it would make sense that a drug that helps people shed pounds would also improve their health, by lowering their cholesterol or blood pressure, for example.
However, back in 2021, Novo didn’t have evidence that that was the case — only that the once-weekly injection led to weight loss. But because Medicare and many insurance companies don’t cover weight-loss drugs, some people have been paying up to $1,350 per month for Wegovy out of pocket.
The weight-loss drug reduced the risk of a serious cardiovascular event by 20%.
The trial: Novo is hoping that SELECT — a huge phase 3 trial testing the ability of Wegovy to reduce the risk of major adverse cardiovascular events (MACE), such as heart attacks, strokes, and death from heart disease — will provide the proof that Wegovy isn’t just a “lifestyle” drug and really does improve people’s health.
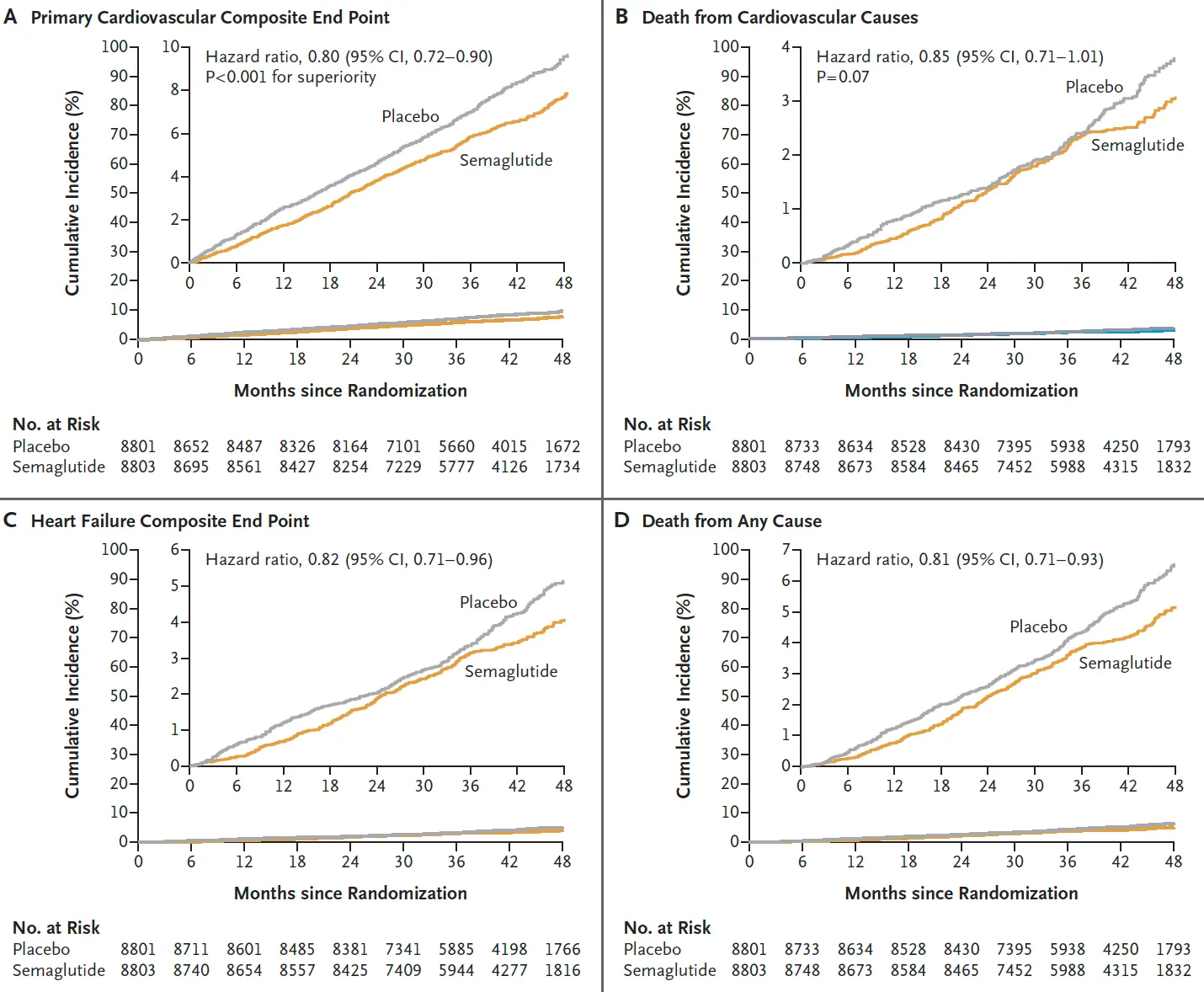
In August 2023, the company reported that the weight-loss drug reduced the risk of MACE by 20% over a period of up to five years during the trial, which included more than 17,500 non-diabetic people with obesity or overweight and a history of heart disease.
Those were just the preliminary trial results and details were scarce. Now, Novo has published the SELECT data in the New England Journal of Medicine (NEJM), while also presenting the findings at a meeting of the American Heart Association.
Wegovy might be improving health in ways that aren’t strictly linked to weight loss.
The details: Based on the new data, we now know that the 20% reduction in the risk of MACE was not dependent on age, gender, ethnicity, or body mass index (BMI) at the start of the trial — all demographics benefited equally.
The reduced risk was not equal for each type of event, though — it dropped by 28% for non-fatal heart attacks, 15% for death from heart disease, and 7% for non-fatal strokes. Patients who got Wegovy also had a 19% lower risk of death from any cause.
Patients given the drug lost about 9% of their body weight, compared to just 1% in the placebo group, but participants started seeing a reduced risk of MACE very soon after treatment, before they had time to lose significant weight.
According to Novo, this suggests that Wegovy — which was originally formulated as a diabetes drug — might be improving health in ways that aren’t strictly linked to weight loss, though more research would be needed to explore that.
The cold water: A 20% risk reduction in MACE would be huge if the risk was very high — if, say, 50% of the people in the placebo group experienced MACE. That would mean treating 100 people with Wegovy prevents 10 of them from experiencing a MACE.
In actuality, these events weren’t that common during the trial, even though the patients were selected to be high-risk — just 8% of the placebo group experienced a MACE over a mean of 40 months, compared to 6.5% of the treatment group. That means treating 100 people with Wegovy for more than three years would help just 1.5 people avoid heart attack, stroke, or cardiovascular death.
That’s a lot of people to treat to prevent relatively few major health events, especially when you consider the high cost of the drug, its unpleasant common side effects, and the potential for it to cause stomach paralysis. (About 17% of patients in the Wegovy group stopped taking the drug due to adverse effects, compared to 8% of the placebo group.)
“The results from SELECT will be instrumental in changing the way we perceive and treat obesity.”
Martin Lange
Still, while MACE are among the most serious health issues linked to obesity or overweight, they’re not the only ones, and compared to the placebo group, treated trial participants saw greater improvements in cholesterol and blood pressure levels. They were much less likely to become diabetic, too.
Looking ahead: Novo has asked the FDA to update its Wegovy label to include a reduced risk of MACE in people with established cardiovascular disease, based on the SELECT results. The agency recently agreed to prioritize the review of the request and is expected to make a decision in 2024.
If the label is updated, it could encourage insurers to start covering the weight-loss drug, putting it within reach of more of the people with obesity or overweight who could benefit from it.
“This landmark study builds on more than 20 years of research in obesity, a serious chronic disease associated with severe co-morbidities and outcomes,” said Martin Lange, executive VP and head of development at Novo Nordisk. “The results from SELECT will be instrumental in changing the way we perceive and treat obesity.”
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].