A smile is always in style (even at funerals), and nothing boosts confidence like a mouth full of healthy, pearly white teeth. Unfortunately, however, “healthy” and “pearly white” rarely go hand in hand. Brushing doesn’t remove deep stains, and whitening treatments can erode the enamel, increasing the risk of cavities and, ironically, discoloration.
Fortunately, a recent study in ACS Applied Materials & Interfaces reports a new nanoparticles treatment that whitens teeth without damaging them, and also protects them by removing cavity-forming bacteria.
The vicious circle of tooth decay and discoloration
In 2019, the Institute for Health Metrics and Evaluation published the most comprehensive global study of health: the Global Burden of Disease 2019 study. Researchers analyzed 286 causes of death, 369 diseases and injuries, and 87 risk factors in 204 countries and territories. According to this study, the most common health condition is tooth decay — erosion of the tooth often caused by sticky, acidic plaques of bacteria.
The first step toward preventing tooth decay is removing these acidic plaques. Brushing your teeth is a good start, but it can’t remove all the bacteria. Eventually, ultra-sticky plaque will cover your teeth and need to be removed by a professional.
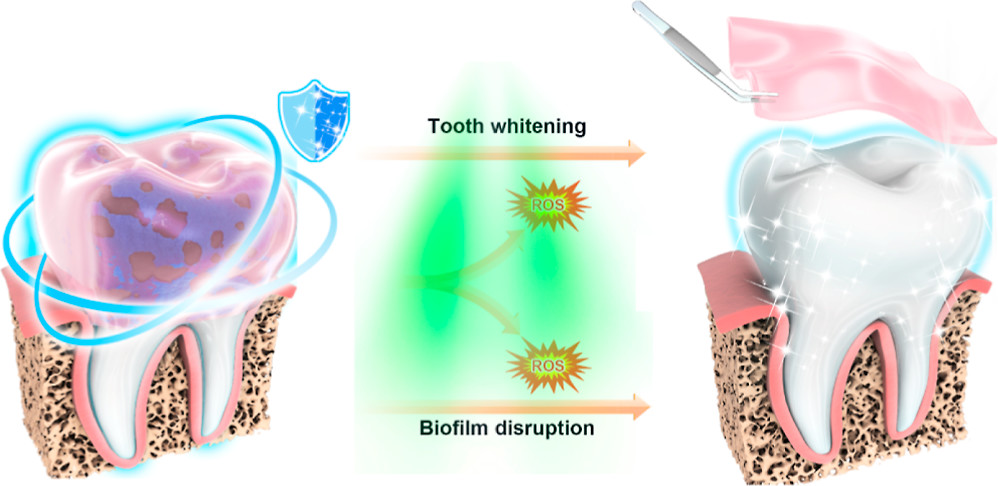
Unfortunately, not everyone has access to a professional. So, oral engineers are always on the prowl for treatments that destroy stubborn plaques. The problem with such therapies is that they can cause side effects, including discoloration of the teeth after long-term use. These stains can be removed through clinical whitening methods using high-intensity blue light and hydrogen peroxide. But blue light can cause damage to the skin and eyes, and hydrogen peroxide’s strong anti-staining power also causes it to be highly corrosive. In other words, compounds that remove plaque-causing bacteria can stain teeth, and treatments that remove stains can damage the tooth surface, allowing bacteria to attach more easily. It is a vicious cycle of tooth decay and discoloration.
Replacing your toothpaste with a nanozymes hydrogel
A few years ago, oral health bioengineers began exploring the possibility of removing plaques with iron oxide nanoparticles. One biomedical material ─ bismuth oxychloride (Bi12O17Cl2) ─ showed promise. Scientists found that they could precisely control its activation using green light instead of blue. Once activated, it could whiten teeth and destroy plaques. But unfortunately, bismuth oxychloride doesn’t stick to teeth long enough to be effective.
Xiaolei Wang and his colleagues at Nanchang University wanted to figure out a way to make the bismuth oxychloride nanoparticles adhere to teeth longer. Their solution was to add another nanoparticle. Cuprous oxide (Cu2O) has broad-spectrum antibacterial properties, is activated by green light, and forms a hydrogel when mixed with bismuth oxychloride and calcium.
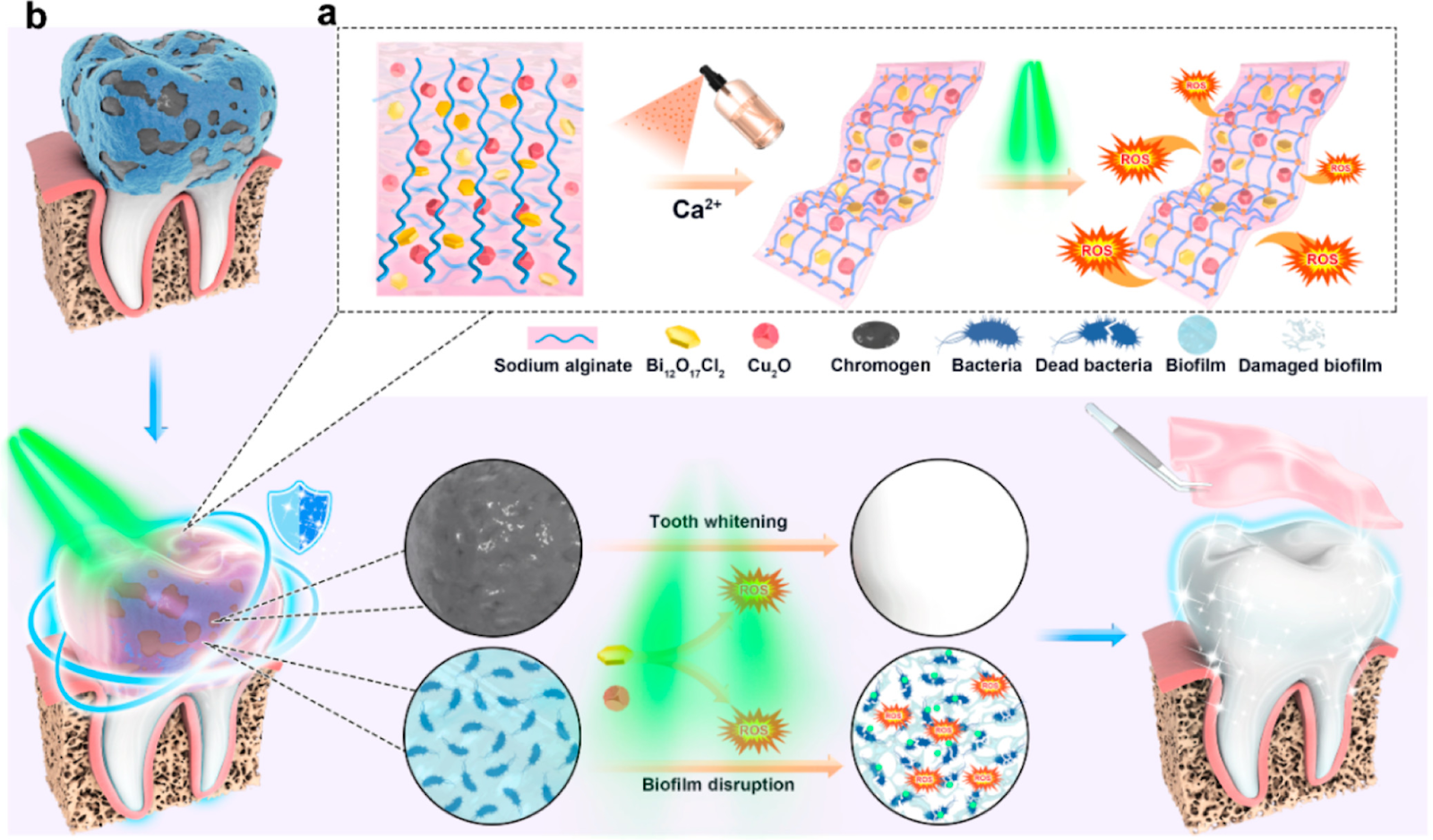
Nanoparticle hydrogel whitens teeth and destroys plaques
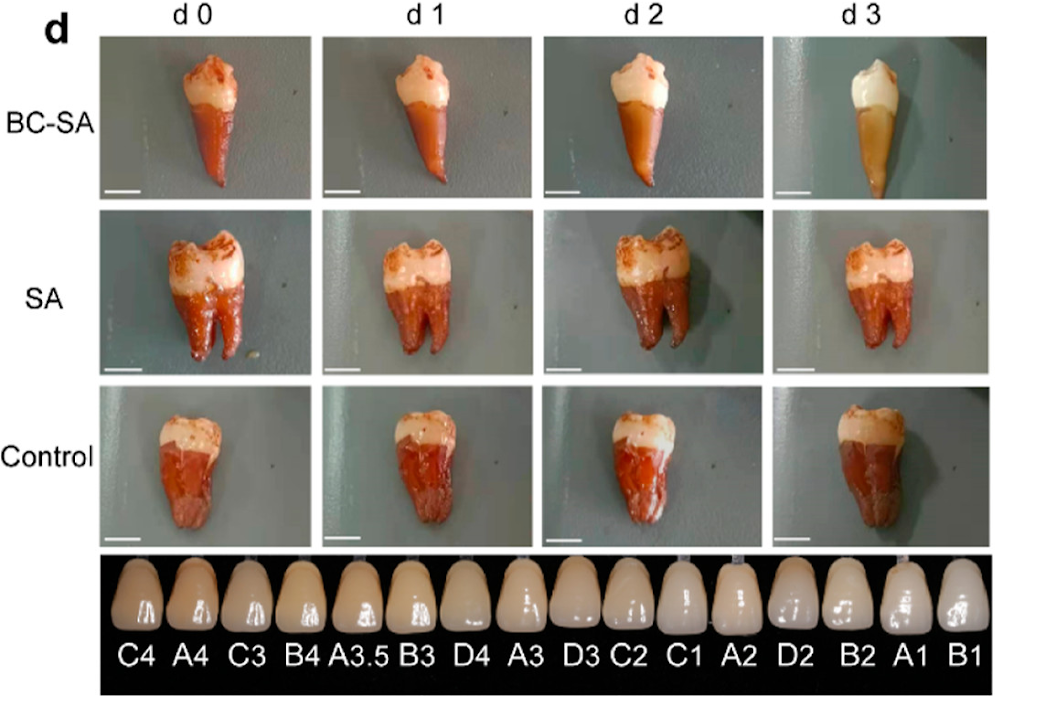
The team tested the material on human teeth soaked in a mixture of coffee, tea, blueberry juice, and soy sauce for 15 days to simulate natural teeth coloration. Next, the teeth were coated in hydrogel and exposed to green light for 1 hour daily. Following treatment, the teeth got brighter over time. Additionally, the treatment caused no damage to the enamel and killed 94% of bacteria in biofilms.
For many of us, cleaning our teeth in a petri dish and exposing them to green light (GL) for an hour each day is not very practical. The team used mice whose mouths were inoculated with cavity-forming bacteria to demonstrate that the treatment worked on teeth in a living organism. The hydrogel was applied to the tooth surface and treated with five minutes of GL twice daily. After 30 days, the dental plaque area was significantly smaller after treatment, accounting for only 9% of the total tooth area.
In humans, if the plaque area accounts for less than 20% of the tooth, it is considered under control. Furthermore, the green-light-activated hydrogel effectively prevented moderate and deep cavities from forming on the surface of the animal’s teeth (despite the lack of brushing).
The researchers concluded their brush-free treatment prevents cavities and whitens teeth. However, there are still some deficiencies in this study. For example, the viscosity of BC-SA can be improved, and the long-term stability of BC-SA needs further experimental exploration. Nevertheless, this is a hopeful step forward in improving oral health.
This article was reprinted with permission of Big Think, where it was originally published.