A new delivery system for prime editing has been used to correct blindness-causing mutations in mice — a promising sign that it could one day be used to treat genetic disorders in people.
Prime editing 101: CRISPR is revolutionizing healthcare, agriculture, and more, but it’s still a flawed tool.
The standard CRISPR system makes edits by snipping through both strands in a DNA molecule, like scissors. Sometimes these cuts end up in the wrong place, and as cells attempt to repair the breaks, they can introduce unwanted mutations.
“In principle, [prime editing] could correct up to 89% of known genetic variants associated with human diseases.”
Liu et al. 2019
Prime editing is a more precise, versatile CRISPR technique developed by David Liu’s lab at the Broad Institute of MIT and Harvard. It cuts through just one strand of DNA during editing and is less likely to lead to off-target edits.
“Prime editing substantially expands the scope and capabilities of genome editing, and in principle could correct up to 89% of known genetic variants associated with human diseases,” Liu’s team wrote in a 2019 paper.
The idea: The most popular delivery vehicles for CRISPR systems — adeno-associated viruses (AAVs) — are too small to hold prime editors, though, so finding an efficient way to get them into cells is key to realizing their potential.
Virus-like particles (VLPs) are one possible alternative. VLPs look like viruses and are made of viral proteins, but they lack any viral genetic material, so they can’t replicate or infect cells. This has made them useful ways to package and deliver vaccines.
In 2022, Liu’s team modified the structure of a VLP so that it would be an efficient delivery system for a base editor (another kind of CRISPR tool). They’d hoped the engineered VLP (eVLP) would work for prime editors, too, but it didn’t — in the experiment, almost no cells were successfully edited.
The system edited up to 20% of the rodents’ retina cells — enough to partially restore their vision.
What’s new? For the new study, the Broad group re-engineered their eVLP and made tweaks to two parts of their prime editor: the Cas9 protein (the scissors) and the RNA (which guides them to the right place). They then painstakingly combined the two so that the prime editor would fit in the particle, but not get stuck in there when it needs to get out and edit the genes.
“The prime editor cargo must be efficiently packaged into eVLPs when the particles form but must also be efficiently released from the particles after target cell entry,” said study co-author Aditya Raguram. “All of these steps have to be carefully orchestrated in order to achieve efficient eVLP-mediated prime editing.”
The results: Compared to a standard prime editor packed into the original eVLP, the new combo was 170 times more efficient at editing human cells.
To test the system in vivo, Liu’s team programmed modified prime editors to correct two different mutations that both lead to vision loss. When tested in mouse models, the systems edited up to 20% of their retina cells — enough to partially restore their vision.
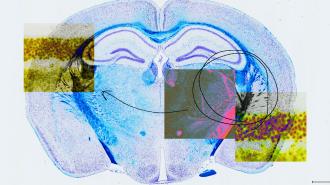
The researchers also tested their new system in the brains of mice and found that it edited nearly 50% of the cells in the target region without making any unwanted edits — all promising signs that this approach could help overcome prime editing’s delivery woes.
“This study represents the first time to our knowledge that delivery of protein-RNA complexes has been used to achieve therapeutic prime editing in an animal,” said Liu. “We plan to continue to actively work on improving eVLPs and adapting the technology to target other tissue types within the body.”
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].