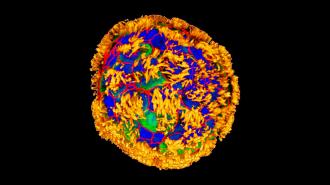
Tiny “biobots” made from human windpipe cells encouraged damaged neural tissue to repair itself in a lab experiment — potentially foreshadowing a future in which creations like this patrol our bodies, healing damage, delivering drugs, and more.
The background: In a study published in 2020, researchers at Tufts University and the University of Vermont (UVM) harvested and incubated skin cells from frog embryos until they were tiny balls.
They then sculpted the spheres into specific shapes — dictated by an algorithm — and added layers of cardiac stem cells to them in precise locations.
“It’s a new class of artifact: a living, programmable organism.”
Joshua Bongard
When they were done, they had created “xenobots,” assembled from frog cells, that could move around and perform entirely new actions based on their designs — one kind of xenobot would push pellets around a petri dish, for example, while another would spin in circles.
“These are novel living machines,” co-lead researcher Joshua Bongard said at the time. “They’re neither a traditional robot nor a known species of animal. It’s a new class of artifact: a living, programmable organism.”
These biological robots might one day navigate the human body, delivering drugs, performing surgery, clearing plaques from artery walls, and more, the team hypothesized in its study.
In 2021, the researchers updated their xenobots to be faster, have memories, and no longer require heart cells. But a major hurdle still stood between the group and its dream of using the biobots in medicine: they were made from frog cells.
That meant the xenobots would likely trigger an immune response in a person, and while immunosuppression could prevent this, it leaves patients at high risk of infection.
Anthrobots, assemble: Building on this xenobot research, Tufts scientists have now developed “anthrobots.”
These biobots are derived from adult human cells, meaning a patient could use their own cells and avoid triggering an immune response. The biobots don’t reproduce and biodegrade after about 45-60 days in the lab, which would further reduce risks to patients.
“It’s fully scalable. We can produce swarms of these bots.”
Gizem Gumuskaya
Anthrobots are also easier to make than their predecessors.
“Anthrobots self-assemble in the lab dish,” said study co-author Gizem Gumuskaya. “Unlike xenobots, they don’t require tweezers or scalpels to give them shape, and we can use adult cells — even cells from elderly patients — instead of embryonic cells.”
“It’s fully scalable,” she continued. “We can produce swarms of these bots in parallel, which is a good start for developing a therapeutic tool.”
How it works: To create an anthrobot, the researchers start with a single cell taken from the surface of a person’s trachea. These cells in your windpipe are naturally covered in tiny, hair-like structures called “cilia” that help keep particles you breathe in out of your lungs.
This single cell is then placed in an environment resembling the human trachea and allowed to grow and multiply for 2 weeks.
At that point, the Tufts team only has a spherical clump of cells with all its cilia on the inside, so they place it in a solution that causes the hair-like structures to move to the exterior. Within days, the cilia start acting like oars, propelling the anthrobot around in its petri dish.

While no two anthrobots were exactly alike, the Tufts team discovered that they tended to assemble themselves into certain categories. Some were spherical, covered in cilia, and wiggled largely in place. Others were football-shaped, with patches of cilia — those tended to move horizontally for longer stretches.
These different shapes and capabilities could potentially lend themselves to different types of therapies in the future.
Neural repair: As a test of the little biobots, the Tufts team created a 2D layer of neurons in a petri dish and then scratched it with a metal rod. They then place a dense concentration of anthrobots on part of the “wound.”
Surprisingly, their presence seemed to encourage healing — new neurons grew below the anthrobots, but not on gaps that were left uncovered.
“It is fascinating and completely unexpected that normal patient tracheal cells, without modifying their DNA, can move on their own and encourage neuron growth across a region of damage,” said study leader Michael Levin, director of Tufts’ Center for Regenerative and Developmental Biology.
“We’re now looking at how the healing mechanism works, and asking what else these constructs can do,” he continued.
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].