If you’ve looked in your utility drawer lately, you may have noticed the various shapes, sizes and types of batteries that power your electronic devices. First, there are the round, non-rechargeable button cells for your watches and small items. There’s also the popular AA and AAA cylindrical batteries for calculators, clocks and remotes. Then you have the rechargeable lithium-ion batteries in your laptops and phones. And don’t forget about the lead-acid battery in your car.
I’m a professor who studies batteries and electrochemistry. To understand why batteries come in many different sizes and shapes – and serve many purposes – look to the past, at how batteries originated and how they have developed over the years.
The first batteries were made in the 1800s, and they were quite simple. One of the first demonstrations was a series of metal discs soaked in brine, which Italian scientist Alessandro Volta found created an electric current. The first lead-acid battery was made of a few pieces of lead in a jar of sulfuric acid. The modern versions are not that different. They’re just easier to manufacture and contain various additives to improve performance.
In all cases, batteries perform in the same manner: a voltage difference between two dissimilar electrodes produces an electric current, which can be discharged to power a device. Rechargeable batteries can then reverse this current to charge back up. Inside the battery, the electric current is accompanied by the flow of ions through a liquid, the electrolyte.
The passage of each electron in the current is accompanied by the transport of one ion through the electrolyte. Electrodes that can store more ions lead to batteries that can hold more charge and therefore last longer on a single charge. Electrodes that are engineered for faster ion storage lead to batteries that can discharge faster, for high-power applications. Lastly, being able to charge and discharge many times without degrading leads to batteries with long lifetimes.
Lead-acid batteries
The lead-acid battery was the first rechargeable battery invented back in 1859 by Gaston Plante, who experimented with lead plates in an acidic solution and found that the flow and storage of electric current could be reversed.
A lead-acid battery has to be big enough to provide enough charge to start a car. It also has to be usable in cold climates and last many years. Since the electrolyte is a corrosive acid, the external casing has to be tough to protect people and car parts from any possible harm. Knowing all this, it makes sense that modern lead-acid batteries are blocky and heavy.
Alkaline batteries
On the other hand, household devices like calculators and digital scales can afford to use smaller batteries because they don’t require a lot of charge. These are primarily non-rechargeable alkaline batteries that have been used for decades. The standardized cell sizes are AAAA, AAA, AA, C and D, as well as button and coin cells and many others. The sizes are related to how much charge they store – the bigger the battery, the more it holds – and the sizes of the devices they power.
Sometimes, you may find alkaline batteries sold in rectangular shapes, like common 9-volt batteries, but open the outer casing and you’ll find that they are simply a few cylindrical cells connected together inside. Cylindrical batteries have been around so long and used so widely that it just doesn’t make sense for the companies to manufacture anything different – it would require an investment to change their manufacturing facilities, something they’d rather not do.
Lithium ion batteries
Nickel-cadmium batteries were the first widely used rechargeable batteries for household electronics and were popular through the end of the 20th century. But they had their pitfalls. Cadmium is very toxic, and the batteries suffered from a “memory effect,” which decreased their lifetime.
For many decades, lithium was studied for potential use in rechargeable batteries because of its unique properties as a lightweight metal that stores a lot of energy. Sony first commercialized the lithium-ion battery in 1991.
The company made cylindrical cells because these were the easiest to manufacture. In the 1990s, Sony was making lots of camcorders and tapes, and thus had lots of equipment for roll-to-roll manufacturing. It was natural to repurpose this equipment to produce rolls of battery electrodes, which are made by casting films on sheets of copper or aluminum and then rolling them up into a “jelly roll” cylinder.
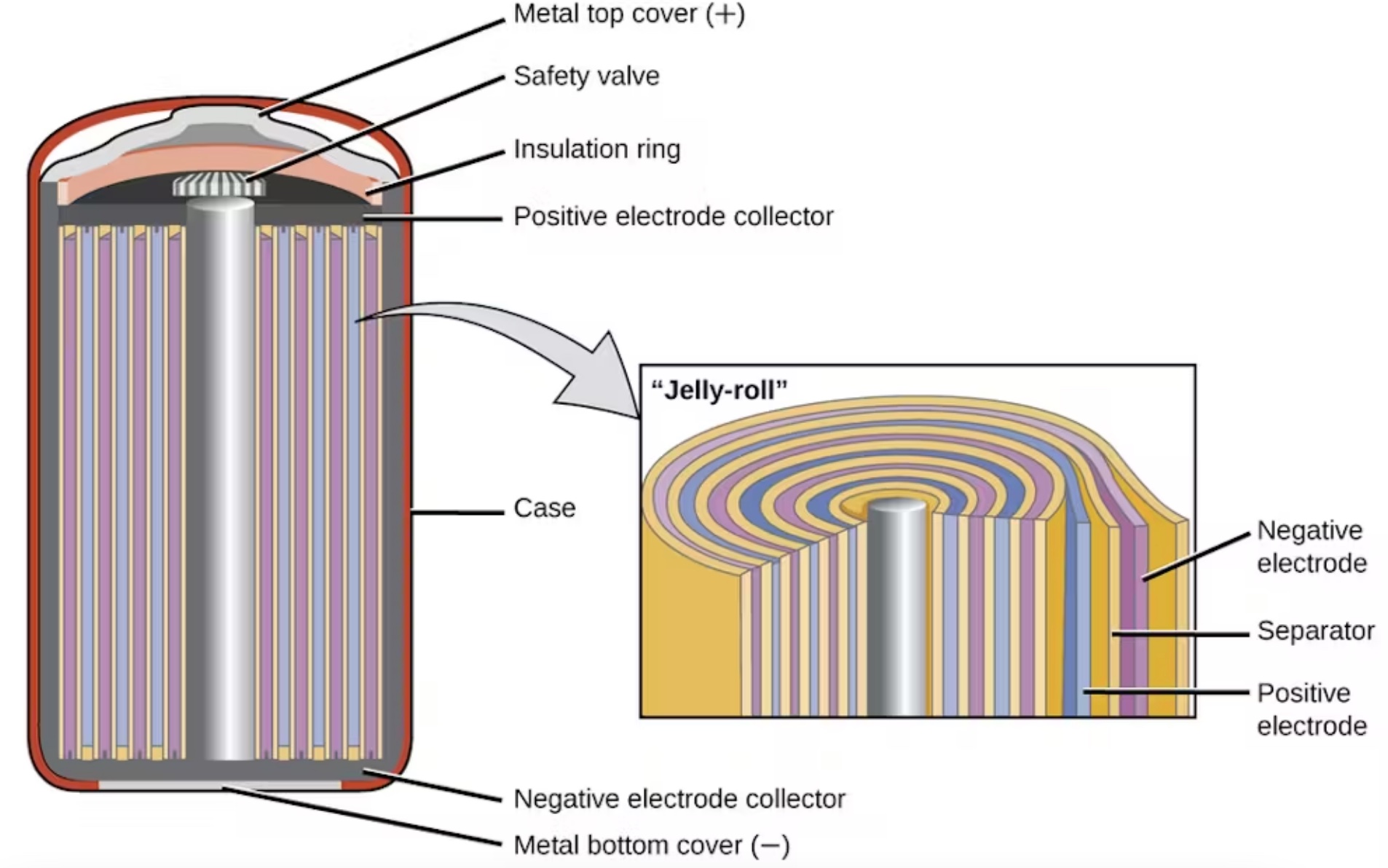
The thick casing of these cylindrical cells is mechanically strong, and to add another layer of safety they have a pressure relief valve. Very quickly, these early lithium-ion cells took over the portable electronics market, especially for laptops and cellphones, because they stored more energy and lasted longer than the nickel-cadmium rechargeable batteries.
Factors that shape batteries
Batteries are made in certain sizes and shapes for reasons of cost and manufacturability, but in other cases because of legacy manufacturing processes. Market demand also plays a role.
For example, electric vehicles didn’t take off until Tesla started making cars using cylindrical lithium-ion battery cells rather than the rectangular pouch or prismatic cells other EV makers have used. Pouch and prismatic cells can be packed closely together, but because cylindrical cells were already being mass-produced for portable electronics, Tesla was able to make lower-cost EVs in the 2010s.
What shapes and sizes batteries will take in the future depends not only on how much energy they store, but also on market economics – how easy it is to make each type of cell, how much it costs to make them and what they’re used for. And those factors are a mix of innovation and history.
This article is republished from The Conversation under a Creative Commons license. Read the original article.






