Update, 6/14/22, 6:00 p.m. ET: On June 13, the FDA approved baricitinib (brand name: Olumiant) to treat adults with severe alopecia areata.
“Access to safe and effective treatment options is crucial for the significant number of Americans affected by severe alopecia,” said Kendall Marcus, director of the Division of Dermatology and Dentistry in the FDA’s Center for Drug Evaluation and Research. “Today’s approval will help fulfill a significant unmet need for patients with severe alopecia areata.”
A Yale University-led trial found that a common arthritis drug can work as an alopecia treatment, helping more than one third of people with severe hair loss regrow their hair in 36 weeks.
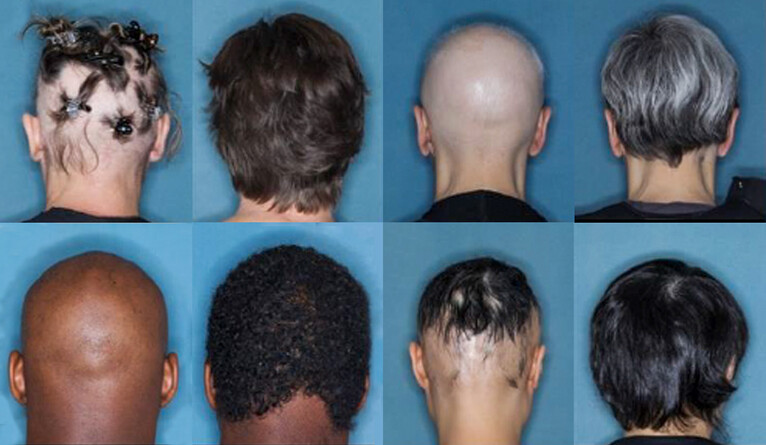
The challenge: Alopecia areata is a disease in which the immune system attacks hair follicles. This causes patchy hair loss, usually on the head or face, but some people experience it on other parts of their body.
In most cases, the hair will grow back on its own, but patients can experience bouts of major hair loss throughout their lives. Some people never regrow their hair, and the FDA has yet to approve any alopecia treatment to help them.
Why it matters: An estimated 1 in 50 people will experience alopecia during their lifetimes, and while hair loss isn’t painful or life-threatening, the disease can affect a person’s self-esteem and lead to depression, anxiety, and other psychological distress.
“Alopecia areata is a crazy journey, marked by chaos, confusion, and profound sadness for many who suffer from it,” said the Yale study’s lead author, Brett King.
Alopecia can affect a person’s self-esteem and lead to depression, anxiety, and other psychological distress.
The idea: Rheumatoid arthritis is another disease caused by an overactive immune system — instead of attacking hair follicles, the immune system attacks the tissue around the joints, causing inflammation.
Baricitinib is a commonly prescribed arthritis treatment that works by tamping down the immune system — and King and his colleagues have now reported that the medication could be an effective alopecia treatment, too.
The trial: The team’s phase 3 trials involved 1,200 people with severe alopecia areata, meaning they’d lost at least half of the hair on their scalp to the disease. For 36 weeks, the participants received either 4 mg of baricitinib, 2 mg of the drug, or a placebo.
The goal was to have participants score 20 or lower on the Severity of Alopecia Tool (SALT), a commonly used tool for measuring hair loss (0 is no scalp hair loss, 100 is complete baldness).
Everyone in the trial started with a SALT score above 50, but by the end of it, more than a third of people in the 4 mg treatment group had scores below 20, compared to only about 5% in the placebo group.
Looking ahead: The phase 3 trials are still ongoing so that the researchers can assess the long-term effectiveness and safety of baricitinib as an alopecia treatment. This is particularly important for any treatments that suppress the immune system, as they have the potential to interfere with the body’s ability to combat infections.
If all goes well, the next step would be seeking FDA approval. In 2020, the FDA designated baricitinib as a “breakthrough therapy” for alopecia after seeing promising phase 2 trial results, which means its final review process should go faster than normal.
“This is so exciting, because the data clearly show how effective baricitinib is,” King said. “These large, controlled trials tell us that we can alleviate some of the suffering from this awful disease.”
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].






