Researchers in China have grown mostly human kidneys in pig embryos. This is the first example of solid human organs being grown in another species — and it could be a turning point in the organ shortage.
The challenge: More than 100,000 people are on the US organ transplant waiting list, and every day, about 17 of them die because a life-saving organ (usually a kidney) wasn’t available in time — the supply of donors simply doesn’t match the demand.
Some researchers have been exploring xenotransplantation — transplanting organs from animals (usually pigs) into humans. Although there’s been some promising advances recently, there’s a huge challenge here: the human immune system often attacks these foreign organs.
Every day, 17 people die in the US because a life-saving organ wasn’t available in time.
So other scientists are testing a creative workaround, where human organs are grown inside an animal by injecting human stem cells in the right place in an animal embryo.
In theory, this process (called “interspecies organogenesis”) could provide us with an unlimited supply of human organs. We could implant human stem cells into the embryos of animals about the same size as us, such as pigs, and then harvest them from the adult animals.
If a transplant recipient’s own stem cells could be used to grow an organ, they might not even need to take immunosuppressants after the operation, since the organ would be a perfect match.
However, while researchers have managed to grow rat organs in mice, and vice versa, as well as human tissues, such as skeletal muscle, in pigs, no one has achieved the ultimate goal of growing solid human organs in another species yet.
“[K]idneys with a normal structure and 50-60% human cells were extracted, which is very promising indeed.”
Rafael Matesanz
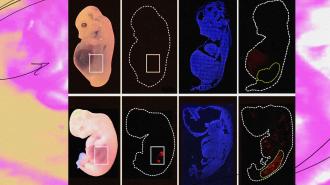
What’s new? Researchers at the Guangzhou Institutes of Biomedicine and Health in China have now come closer to that holy grail than anyone else, reporting in the journal Cell Stem Cell on September 7 that they grew partially human kidneys inside pig embryos.
After growing the embryos for 25-28 days, “kidneys with a normal structure and 50-60% human cells were extracted, which is very promising indeed,” said Rafael Matesanz, creator and founder of Spain’s National Transplant Organization, who wasn’t involved in the study.
How it works: The researchers had to overcome several challenges, the first being the fact that pigs grow their own kidneys, and their kidney cells would outcompete the human stem cells for resources in the embryo.
To surmount that hurdle, they used the gene-editing technology CRISPR to knock out two pig genes essential to kidney development. This created a hospitable microenvironment, or “niche,” for the human stem cells in the embryo and helped them compete with the pig’s own kidney cells.
The human stem cells were also engineered to overexpress two pro-survival genes, and before the embryos were implanted in surrogate sows, they were cultivated in lab conditions optimized for the survival of both human and pig cells.
A small number of human cells were found in the embryos’ brains and spinal cords.
The cold water: Over the course of the study, more than 1,800 embryos were transplanted into 13 surrogates. From all of those, only five successfully implanted and had normally structured kidneys at day 25 or 28. Those were the kidneys in which 50-60% of the cells were human.
That’s a success rate of less than 0.3% — and we don’t know for sure that these kidneys would have continued to develop properly after that.
Getting the success rate up and increasing the percentage of human cells in the kidneys will be essential to making this a viable solution to the organ shortage. That’s likely going to require more genetic engineering and could take many years, according to the researchers.
The team will also need to take steps to ensure only the target organ is “humanized.” In their study, they discovered a small number of human cells in the embryos’ brains and spinal cords, which raises serious concerns about accidentally altering the animals’ mental capacity or overall development.
They didn’t find any human cells in the other place we really don’t want them, though: the animals’ germline tissues (the ones that develop into reproductive organs, sperm, and eggs).
The big picture: Even if scientists do figure out how to grow perfect, 100% human organs in pigs without affecting any other parts of their bodies, the practice of using animals as organ incubators raises ethical questions.
Those same questions surround the transplantation of modified animal organs into people, and that practice is much farther along than interspecies organogenesis. In 2022, a man lived for two months after surgeons replaced his failing heart with one from a genetically engineered pig, and in August 2023, researchers announced that a pig kidney was still functioning 32 days after transplantation into a man who was brain dead and on life-support.
(Those were entirely pig organs, but they had been genetically tweaked to make them look more human and have fewer pig proteins that might trigger rejection by the human immune system.)
We might not need to grapple with the ethics of using animals as a source of organs, though, if other in-development solutions to the shortage pan out first, such as bioartificial organs, lab-grown organs, and treatments that regenerate damaged organs.
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at tips@freethink.com.