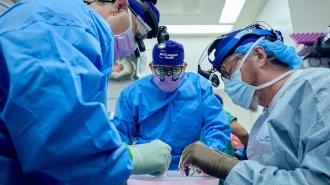
A gene-edited pig kidney has been functioning in a person for a record-breaking 32 days and still shows no signs of failure — another encouraging sign that genetically modified animals may one day end the organ shortage.
The challenge: Nearly 90,000 people are on the waiting list for kidney transplants in the US, and every day, approximately 13 people die because a donor organ wasn’t available in time.
If we could source kidneys from animals, we’d have a virtually unlimited supply. Getting the body to accept an organ from another human is already a challenge, though, and preventing the rejection of animal organs has historically proven impossible.
Every day, 13 people die because a donor kidney wasn’t available in time.
What’s new? To solve this problem, regenerative medicine company Revivicor is using gene-editing technology to modify pigs so that their organs are less likely to be rejected by the human body.
On July 14, surgeons at NYU Langone Health transplanted a Revivicor pig kidney into a man who was brain dead and on life-support, after his family made the decision to donate his body to science. The man’s kidneys were removed during the surgery, but the pig kidney quickly took over, producing urine immediately.
Thirty-two days later, it was still functioning with no signs of rejection.
The advance: This isn’t the first time a pig kidney from Revivicor has been transplanted into the body of a person who is brain dead, but other studies ended after a maximum of one week — the NYU Langone team hopes to keep monitoring this patient until mid-September.
“We think using a pig already deemed safe by the FDA…gets us closer to the clinical trial phase.”
Robert Montgomery
Additionally, while other teams used kidneys from pigs with up to 10 genetic modifications, the NYU Langone team’s kidney came from a GalSafe pig.
Those animals feature just one modification — the elimination of the gene that encodes for alpha-gal, a biomolecule thought to be the cause of rejection in transplant recipients — and the FDA has already approved them for biomedical uses, such as the creation of blood thinners.
“We think using a pig already deemed safe by the FDA, in combination with what we have found in our xenotransplantation research so far, gets us closer to the clinical trial phase,” said lead surgeon Robert Montgomery. “We know this has the potential to save thousands of lives, but we want to ensure the utmost safety and care as we move forward.”
The big picture: In addition to these promising kidney studies, the NYU Langone team has also transplanted hearts from Revivicor pigs into people.
One of those studies took place in early 2022, when a man with no other options was given special permission from the FDA to undergo a pig-to-human heart transplant. He then lived with a Revivicor pig heart for two months before dying, likely due to a pig virus that went undetected in the organ.
More recently, two brain-dead patients at NYU Langone Health survived for three days with Revivicor pig hearts. The organs likely would have kept beating longer, too — the studies only ended because the patients were taken off life support, as planned from the start.
The FDA is reportedly already close to allowing clinical trials of pig organs in people, and if those trials are successful, they could usher in a future in which no one has to die because a life-saving organ wasn’t available to them in time.
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at tips@freethink.com.