UK regulators have authorized CRISPR Therapeutics’ Casgevy to treat two debilitating blood disorders — making it the world’s first approved CRISPR therapy.
“I hope this represents the first of many applications of this Nobel Prize winning technology to benefit eligible patients with serious diseases,” said Samarth Kulkarni, CEO of CRISPR Therapeutics.
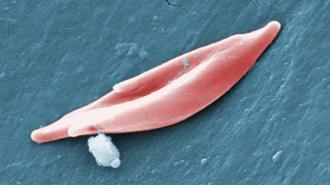
The targets: Your cells need oxygen to function, and it’s the job of hemoglobin, a protein found in your red blood cells, to deliver it.
Sickle cell disease (SCD) and beta thalassemia (BT) are genetic disorders disrupting hemoglobin production. In SCD, the body produces abnormal hemoglobin that causes red blood cells to be rigid, sticky, and curved, or sickle shaped, instead of round — these cells aren’t as effective at carrying oxygen. In BT, the body doesn’t produce enough hemoglobin, period.
The only known cure for SCD or BT is a risky stem cell transplant.
With both diseases, the body doesn’t get as much oxygen as it needs — this can lead to fatigue and weakness. With SCD, blood cells can build up and get stuck in tiny blood vessels, causing episodes of severe pain and swelling. In severe cases, patients can experience organ damage, infections, and blindness.
Medications or frequent blood transfusions can alleviate symptoms, but the only known cure for either disease is a risky stem cell transplant.
The procedure requires patients to undergo chemotherapy to kill their own faulty stem cells, and after they’ve been replaced with transplanted cells, they must take immunosuppressants that leave them at high risk of infection. This process can cause infertility and organ damage — and the transplant doesn’t always work.
The idea: Fixing the mutations that cause SCD or BT is not a simple problem — but there’s a backup option for making hemoglobin, hidden in the human genome.
Fetuses produce a special form of hemoglobin that binds more strongly to oxygen than adult hemoglobin. Production of this fetal hemoglobin (hemoglobin F) typically stops soon after birth, and production of adult hemoglobin (hemoglobin A) takes over.
In trials, 97% of treated SCD patients were pain-free for at least 12 months.
Some people have high levels of hemoglobin F into adulthood, though, and in people who also happen to have SCD or BT, it leads to very mild symptoms. That’s because the mutations that cause SCD and BT affect adult hemoglobin, but not fetal hemoglobin.
In 2008, researchers discovered that specific variants of the BCL11A gene were responsible for high levels of fetal hemoglobin in adults — this gene ordinarily seems to serve as a brake for hemoglobin F production in adulthood, and these mutations release the brake.
The CRISPR therapy: CRISPR Therapeutics’s Casgevy (also known as “exa-cel”) treats SCD and BT by deactivating the BCL11A gene to restart hemoglobin F production.
The process begins by removing some blood-producing stem cells from the patient’s own bone marrow. A CRISPR system is then used to cut the BCL11A gene to deactivate it. Meanwhile, the patient undergoes chemotherapy to kill their remaining stem cells.
The CRISPR-edited cells are then infused back into the patient. Because the cells came from their body, they don’t have to take immunosuppressants to avoid rejection. The cells then repopulate the bone marrow, and hemoglobin F production ramps up.
In clinical trials, 97% of treated SCD patients were pain-free for at least 12 months, and 93% of BT patients — all of whom previously needed blood transfusions every 3-4 weeks — went without transfusions for at least a year.
“I look forward to patients having access to this therapy as quickly as possible.”
Josu de la Fuente
Looking ahead: The MHRA (the UK’s version of the FDA) has approved Casgevy for patients 12 and older with SCD or transfusion-dependent BT and no suitable donor for a stem cell transplant.
The FDA and European regulators are in the process of reviewing the CRISPR therapy, too, so it could soon be available elsewhere.
“This authorization offers a new option for eligible patients who are waiting for innovative therapies, and I look forward to patients having access to this therapy as quickly as possible,” said Josu de la Fuente, principal investigator in two Casgevy studies.
The big picture: Regardless of when Casgevy is approved, access to it will likely be highly restricted, at least initially, due to its estimated $2 million cost. The complexity of the therapy could also put it out of reach of those living in places that lack state-of-the-art healthcare facilities.
Still, now that one CRISPR therapy has proven to be safe and effective, the door is open for others to follow — potentially leading to a future in which we use the groundbreaking gene-editing tool to treat (or cure) HIV, Alzheimer’s, cancer, and more.
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at tips@freethink.com.